that varies a little depending the weight of the wheel but noload speed is a euphemism for derestricted speed that you can easily reach which is a little less than top speed you can see when the bike is on a stand.Woosh my no load speed when i checked is 27.5mph?
Confused by all the choice
- Thread starter Jeremiah ables
- Start date
it does not matter much because the hydraulic brakes have excellent stopping power but sensors are useful when you go round sharp corners or use the throttle.What about my brake aren't they the sensor type ones?
Yes I understand as mine cut the motor when you tap them so they are the sensor ones thenit does not matter much because the hydraulic brakes have excellent stopping power but sensors are useful when you go round sharp corners or use the throttle.
it's easy to see if they have sensors:Yes I understand as mine cut the motor when you tap them so they are the sensor ones then
you have the hydraulic hose and a sensor cable like this:

Last edited:
Yes my Oxygen MTB has the sensor then thought it didit's easy to see if they have sensors:
you have the hydraulic hose and a sensor cable like this:

there, the Oxygen wins 100%.Just for interest, what warranty do you get with the Rio? As as opposed to the Oxygen 5 years on the frame and 2 years on all the electrics and battery?
I've just had a look at the Edge bikes, and notice the front rack mounting points welded on. This is one of the things I have been looking for in my ideal ebike. I notice the central battery, so the weight is in the right place. I just have to wait for one with a front motor, so that I have more choice for the transmission, but I cannot wait too long....... Time is not on my side.Hi Jeremiah,
I bought my first ebike a couple of weeks ago and I absolutely love it. It's much easier to ride than I expected and I could't be more pleased with it. If you go to the web-site and read the specs. I can assure you that the bike has been updated, but not corrected in the specs.
My EDGE.BIKE Hybrid came with hydraulic disc brakes,not mechanical, a 14ah battery not 10ah, mudguards and rear rack. The rest are probably as stated. I can honestly recommend this bike and you should take a look at the web-site, also have a chat with Sam , the man. cheers
View attachment 25677
I notice there is no address for Edge, and the spec given for the bikes is rather limited, when you compare it with the Woosh site and the response you get from Woosh on here. Hmmm...... makes you think.I've just had a look at the Edge bikes, and notice the front rack mounting points welded on. This is one of the things I have been looking for in my ideal ebike. I notice the central battery, so the weight is in the right place. I just have to wait for one with a front motor, so that I have more choice for the transmission, but I cannot wait too long....... Time is not on my side.
As the current rises, the battery's internal resistance increases, QUOTE]
I am no expert on batteries but are you sure about this:? My understanding is that as the current increases the battery will heat up due to increased power losses in the battery due to the internal resistance. The power loss in Watts can be found by multiplying the current squared by the internal resistance.
The internal resistance does not increase as the temperature of the battery increases due to the rising power loss, in fact I think the internal resistance actually starts to decrease as battery temperature increases.
This is why batteries don't last too long on the top of cold mountains, ie. their internal resistance is higher when they are cold.
The plots below show the relationship between internal resistance and discharge current.I am no expert on batteries but are you sure about this:? My understanding is that as the current increases the battery will heat up due to increased power losses in the battery due to the internal resistance. The power loss in Watts can be found by multiplying the current squared by the internal resistance.
The internal resistance does not increase as the temperature of the battery increases due to the rising power loss, in fact I think the internal resistance actually starts to decrease as battery temperature increases.
This is why batteries don't last too long on the top of cold mountains, ie. their internal resistance is higher when they are cold.
The bottom black plot shows the resistance at 0.3A discharge, the red plot just above it the same cell at 0.6A discharge and so on for 0.9A etc.
Clearly, the resistance increases with the discharge current.
The cell internal resistance is the result of friction when Lithium leaves the negative electrode and moves to the positive electrode during discharge. When the discharge current increases, accumulation of discharge products and reduction of concentration of the salts in the electrolyte in the neighbourhood of the electrodes cause congestion and increase the cell internal resistance.
This phenomenon happens not only in Lithium ion battery but is common to a lot of batteries, it is called the Peukert effect.
https://en.wikipedia.org/wiki/Peukert's_law
Typical internal resistance of common Lithium Ion cells:
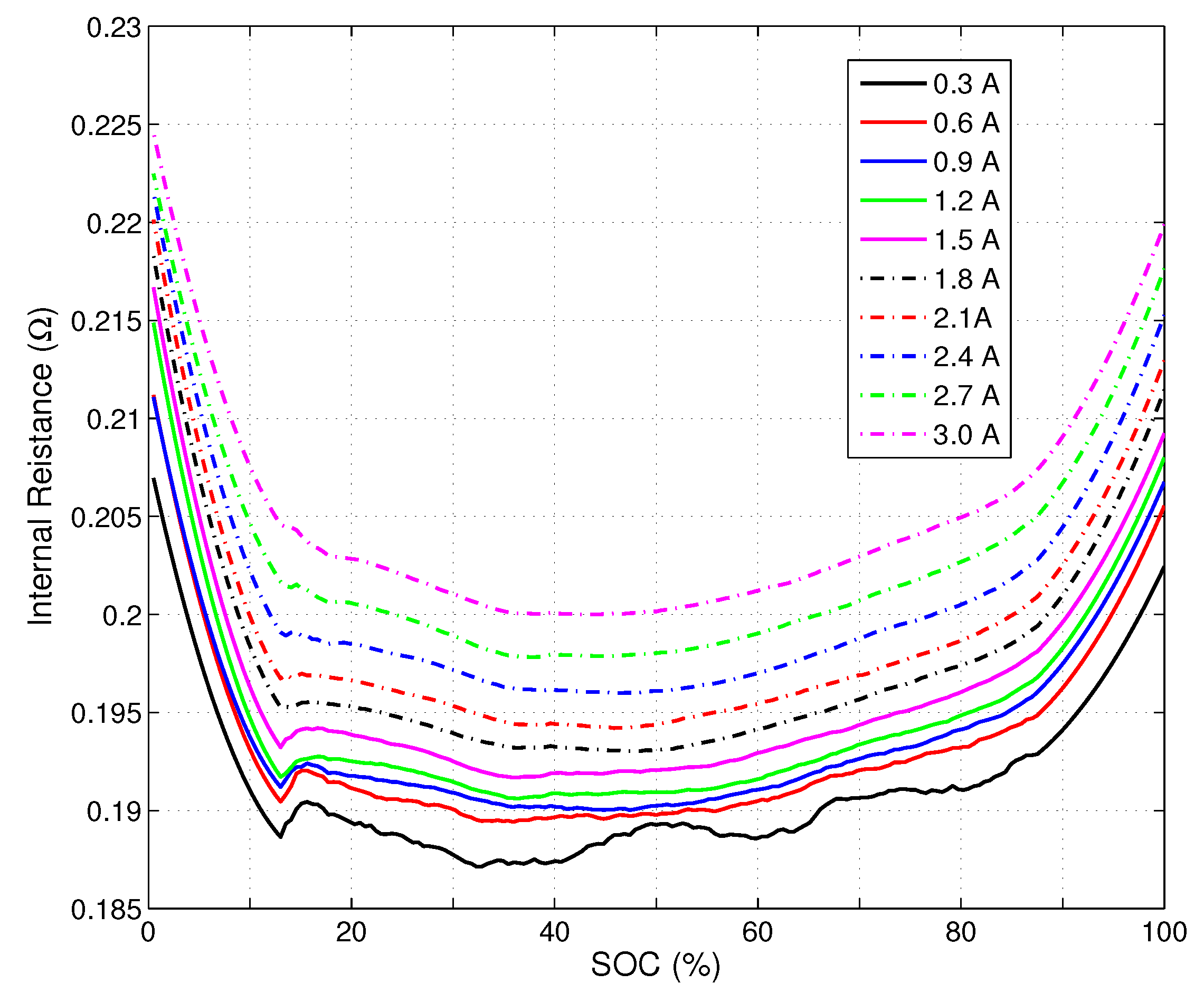
graphs from:
http://www.mdpi.com/1996-1073/6/10/5538/htm
Thanks Woosh, take a look at this what are your thoughts?
https://www.physicsforums.com/threads/why-batterys-internal-resistance-decreases-with-temperature.630648/
https://www.physicsforums.com/threads/why-batterys-internal-resistance-decreases-with-temperature.630648/
Just had another quick look around and found this site which gives lots of info on batteries. Here is a quote from it.
As all drivers in cold countries know, a warm battery cranks the car engine better than a cold one. Cold temperature increases the internal resistance and lowers the capacity.
Here is the site:-
http://batteryuniversity.com/learn/article/discharging_at_high_and_low_temperatures
As all drivers in cold countries know, a warm battery cranks the car engine better than a cold one. Cold temperature increases the internal resistance and lowers the capacity.
Here is the site:-
http://batteryuniversity.com/learn/article/discharging_at_high_and_low_temperatures
yes, your battery internal resistance is higher at 0 deg C than at 20 degrees C but that is due to the construction of Lithium ion cells.Just had another quick look around and found this site which gives lots of info on batteries. Here is a quote from it.
As all drivers in cold countries know, a warm battery cranks the car engine better than a cold one. Cold temperature increases the internal resistance and lowers the capacity.
Here is the site:-
http://batteryuniversity.com/learn/article/discharging_at_high_and_low_temperatures
Your cells are optimised to functioning temperature at 25 degree C. That is, the internal resistance is higher too cold or too hot, lowest between 20 - 40 deg C, then rises again.
Your battery will heat up when you are using it, but you get quickly to an equilibrium, somewhere about 5-10 degrees higher than ambient temperature. This is seen with a 10AH battery, less with greater capacity like 13AH or 17AH battery. The effect of temperature is nevertheless small to insignificant compared to the Peukert effect due to the high discharge current.
Read the datasheet of the Samsung 18650 29E, probably the most popular cell on e-bikes.
https://eu.nkon.nl/sk/k/29E.pdf
Page 4 and page 5 explain the effect of temperature on the cell.
Page 6 explains also the effect of high discharge current on the amount of energy you get, 1C, 2C and 3C: 0.2C: 100%, 1C: 97%, 2C: 95%, 3C: 92%, the reduction is dissipated as heat due to increased internal resistance.
In order to understand why cell performance decreases with discharge current, you need to go back to the Arrhenius Law.
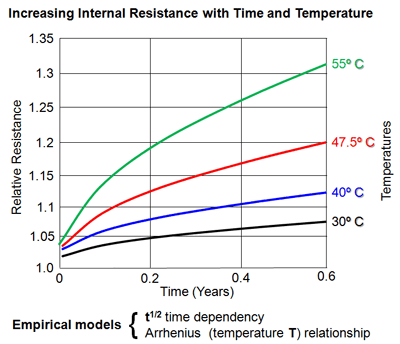
Last edited:
Excellent explanation Woosh thanks for taking the time to answer my questions. Absolutely love this site, so many helpful and knowledgable people here willing to answer our questions.yes, your battery internal resistance is higher at 0 deg C than at 20 degrees C but that is due to the construction of Lithium ion cells.
Your cells are optimised to functioning temperature at 25 degree C. That is, the internal resistance is higher too cold or too hot, lowest between 20 - 40 deg C, then rises again.
Related Articles
-
 MTF Enterprises announces acquisition of EMU Electric Bikes
MTF Enterprises announces acquisition of EMU Electric Bikes- Started by: Pedelecs
-
 Wisper 806T folding bike wins Which? ‘Best Buy’
Wisper 806T folding bike wins Which? ‘Best Buy’- Started by: Pedelecs
-
 Sustrans calls for protected cycle lanes
Sustrans calls for protected cycle lanes- Started by: Pedelecs
-
 Amazon launch their first UK e-cargo micromobility hub
Amazon launch their first UK e-cargo micromobility hub- Started by: Pedelecs


