I repurpose the good cells for torch's or other 18650 gadgets, also I make up smaller 12v packs for which I have other uses for.
Knackered or bad cells either I toss in a bucket of water for a few weeks and then bin them or use them as target practice with my nice air rifle with a scope sight and silencer.
You may want to think that through a bit more, though the reusage for flashlights is a truly excellent idea, that I fully agree with, and I possess several such flashlights!
Though I have up to now, had to buy the cells separately (new), as in spite of around 10 years with two e- bikes and 3 batteries, I have never had a defective battery up to now, of which I am proud, so I must be doing something right!
With regard to defective cells, and what you do with them, especially if you have children/family that live with you:-
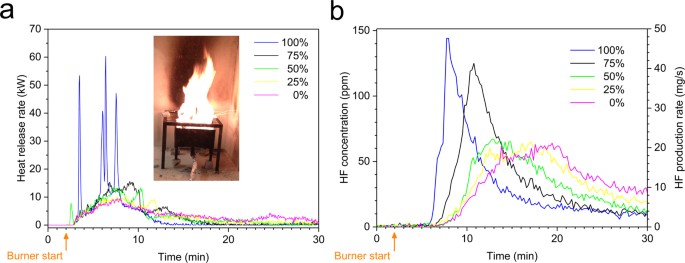
The electrolyte in a lithium-ion battery is flammable and generally contains lithium hexafluorophosphate (LiPF6) or other Li-salts containing fluorine. ... Li-ion batteries release a various number of toxic substances14,15,16 as well as e.g. CO (an asphyxiant gas) and CO2 (induces anoxia) during heating and fire.
Copied from this paper on such cells:-
Lithium-ion battery fires generate intense heat and considerable amounts of gas and smoke. Although the emission of toxic gases can be a larger threat than the heat, the knowledge of such emissions is limited. This paper presents quantitative measurements of heat release and fluoride gas...

www.nature.com
Truthfully, apparently few have made exhaustive tests on just how dangerous these chemicals can be, but the following was noted on that website:-
Lithium-ion battery fires generate intense heat and considerable amounts of gas and smoke. Although the emission of toxic gases can be a larger threat than the heat, the knowledge of such emissions is limited. This paper presents quantitative measurements of heat release and fluoride gas emissions during battery fires for seven different types of commercial lithium-ion batteries. The results have been validated using two independent measurement techniques and show that large amounts of hydrogen fluoride (HF) may be generated, ranging between 20 and 200 mg/Wh of nominal battery energy capacity. In addition, 15–22 mg/Wh of another potentially toxic gas, phosphoryl fluoride (POF3), was measured in some of the fire tests. Gas emissions when using water mist as extinguishing agent were also investigated. Fluoride gas emission can pose a serious toxic threat and the results are crucial findings for risk assessment and management, especially for large Li-ion battery packs.
The use of water mist as an extinguishing agent may promote the formation of unwanted gases as in eqs (2)–(3) and our limited measurements show an increase of HF production rate during the application of water mist, however, no significant difference in the total amount of HF formed with or without the use of water mist.
My idea, mentioned here, to actually sink either the whole battery, or the whole bike in water, just as the German autobahn Police, extinguish electric cars which are burning, with a large tank of water, would appear to be, if at all possible, the best idea. If not possible, just stay far away and seriously "up-wind" of the battery and its emissions. Which begs the question, if there was such a fire in a house, or even in a garden shed, will the building become toxic in some manner?
I also had a quick glance through for long term effects on humans and animals, but found very little useful info.
A full video here, in a US NASA experimental lab, is seriously worrying too:-
The Defense Advanced Research Projects Agency's 'RoboSimian' exploded at Nasa's Jet Propulsion Laboratory on June 14th, the agency confirmed.

www.dailymail.co.uk
The references are apparently a list of serious battery accidents that have been noted,...
I hope that this was at least of some interest for you, do stay healthy.
Regards
Andy

 relionbattery.com
relionbattery.com